Covid-19 vaccine temperature monitoring : requirements and practical solutions
- What are the storage temperatures for Pfizer-BioNTech vaccine?
- What are the requirements of health authorities around the world?
- What solutions to monitor the temperature of refrigerators in pharmacies ?
The first vaccine against Covid-19, Pfizer-BioNTech, has just been approved for use in many countries, including France. Storage temperatures, at -80°C and then between +2 and +8°C, are precise and must be respected to ensure the quality of the vaccination.
Other vaccine candidates will soon be available : Moderna, to be stored at -20°C, Astra Zenecca, Sanofi and others at 2 and 8°C.
Plug and Track, cold chain specialist, has been supplying for more than 20 years temperature monitoring systems for refrigerators in pharmacies and hospitals, allowing them to control the cold chain of vaccines and medicines.
What are the storage temperatures for Pfizer-BioNTech vaccine?
-
-
- The Pfizer-BioNTech vaccine requires storage at -80°C.
- Transport to pharmacies is done at +2/+8°C within a maximum of 12 hours.
- Once delivered to the pharmacy, the vaccine must be stored between +2°C and +8°C.
- The total storage time at +2/+8°C must not exceed 5 days.
-
Storage temperature of Moderna vaccine:
- The vaccine can be stored for 6 months at a temperature between -25 and -15°C.
- After thawing, the vials can be stored in the refrigerator between 2 °C and 8 °C for up to 30 days before first use.
Storage temperature of AstraZeneca
- The storage temperature is between 2 and 8°C.
- Shelf life is six months
What are the requirements of health authorities around the world ?
In all countries, the national authorities for health request an accurate monitoring of temperature during storage of vaccines.
These agencies require that a temperature traceability be carried out. It is also necessary to have a temperature datalogger in order to have :
- Records indicating the minimum and maximum temperatures reached within refrigerators, freezers, cases, etc.
- A history of the different temperatures reached.
In the USA, the Center for Desease Control has published a specific storage and handling guidelines for the Pzifer and the Moderna Vaccines.
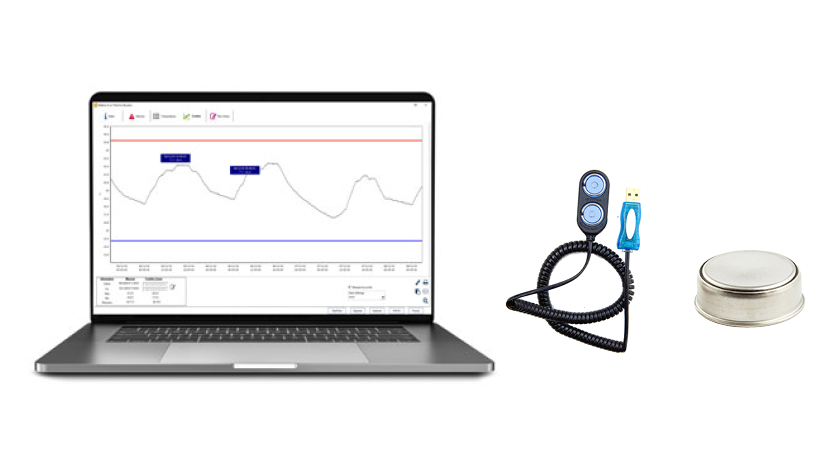
In both cases, it is recommended to use a DDL Digital Data Logger to record temperature. If possible “storage unit temperatures must be monitored regularly and checked and recorded at the beginning of each workday to determine if any excursions have occurred since the last temperature check. For accurate temperature monitoring, use a digital data logger (DDL)”.
Most DDLs display minimum and maximum (min/max) temperatures. Check and record the min/max temperatures at the start of each workday.
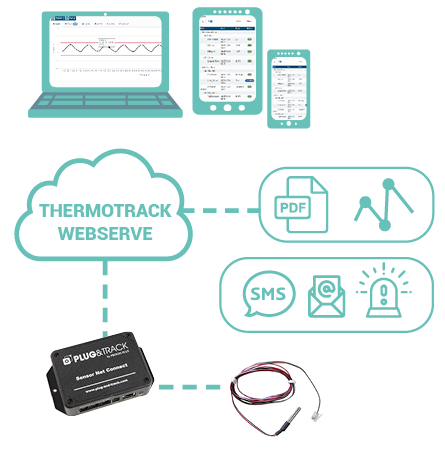
What solutions to control the temperature of refrigerators in pharmacies ?
In order to meet the recommendations of global health authorities Plug and Track, specialist in temperature monitoring and cold chain, offers 2 solutions :
|
 |
|
 |
